[ Recombinant Protein ] Empowering Cytokine-Induced Killer (CIK) therapy
GMP-grade Recombinant Proteins
Empowering Cytokine-Induced Killer (CIK) therapy
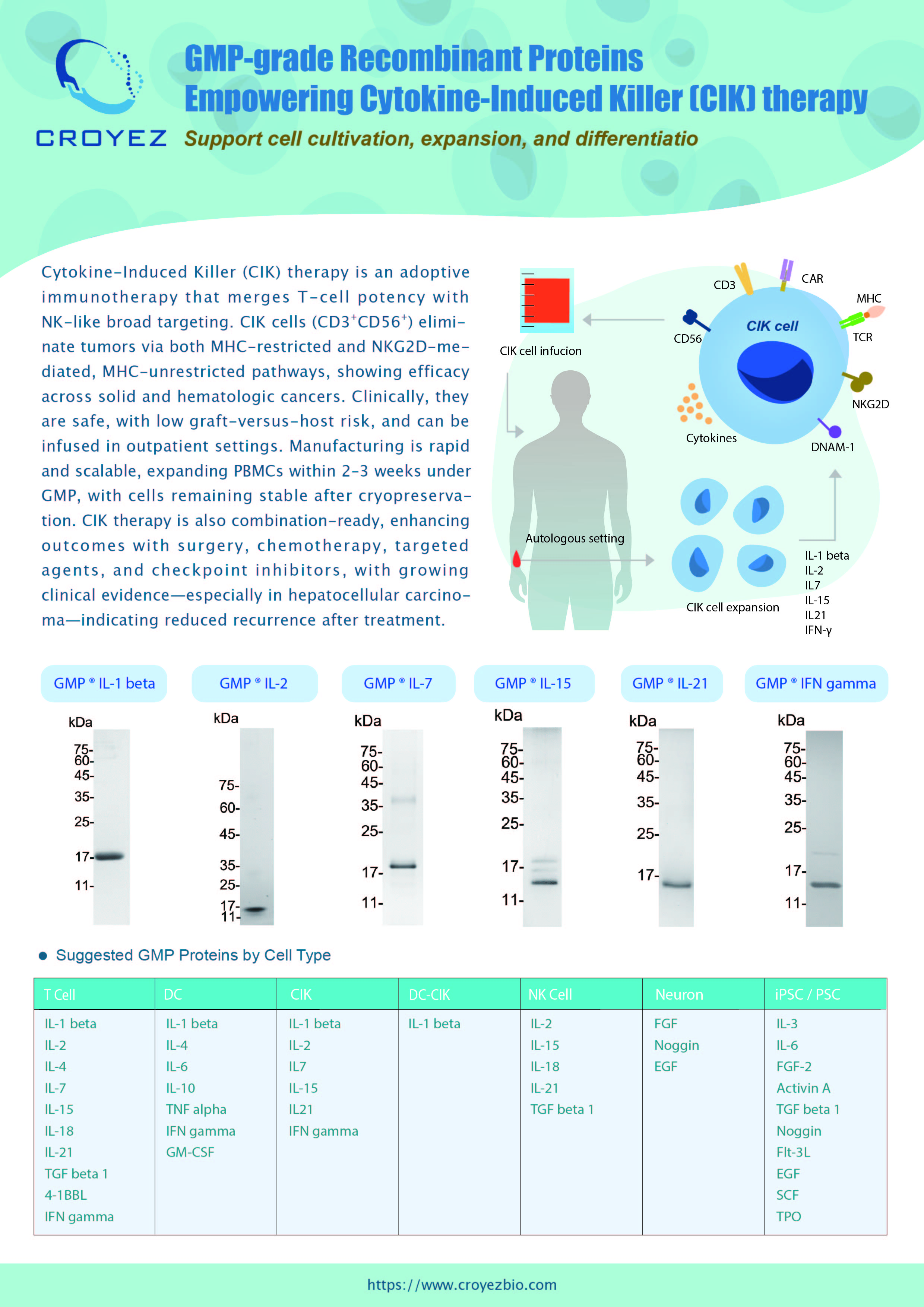
Cytokine-Induced Killer (CIK) therapy is an adoptive immunotherapy that merges T-cell potency with NK-like broad targeting. CIK cells (CD3⁺CD56⁺) eliminate tumors via both MHC-restricted and NKG2D-mediated, MHC-unrestricted pathways, showing efficacy across solid and hematologic cancers. Clinically, they are safe, with low graft-versus-host risk, and can be infused in outpatient settings. Manufacturing is rapid and scalable, expanding PBMCs within 2–3 weeks under GMP, with cells remaining stable after cryopreservation. CIK therapy is also combination-ready, enhancing outcomes with surgery, chemotherapy, targeted agents, and checkpoint inhibitors, with growing clinical evidence—especially in hepatocellular carcinoma—indicating reduced recurrence after treatment.
PRODUCTS :
GMP Grade Recombinant Protein ➡︎