GMP® 4-1BBL, Human
4-1BB Ligand Human Recombinant protein. 4-1BBL is a transmembrane cytokine that is part of the tumor necrosis factor (TNF) ligand family. Recombinant human 4-1BB ligand is intended for use in cell culture applications. 4-1BBL and its interaction with 4-1BB is involved in the antigen presenting process, proliferation of CD4 and CD8 positive T-cells, as well as cytokine secretion from T-cells.
✔️ Scarce GMP-certified Quality
✔️ Over 95% purity
✔️ Effectively stimulate and expand NK cells
✔️ Scarce GMP-certified Quality
✔️ Over 95% purity
✔️ Effectively stimulate and expand NK cells
Sequence
MREGPELSPDDPAGLLDLRQGMFAQLVAQNVLLIDGPLSWYSDPGLAGVSLTGGLSYKEDTKELVVAKAGVYYVFFQLELRRVVAGEGSGSVSLAL
HLQPLRSAAGAAALALTVDLPPASSEARNSAFGFQGRLLHLSAGQRLGVHLHTEARARHAWQLTQGATVLGLFRVTPEIPAGLPSPRSE
with polyhistidine tag at the C-terminus
UnitProt ID
P41273
Species of Origin
Human
Expression System
Escherichia coli
Endotoxin Level
<0.05 EU per 1 μg of the protein by the LAL method.
Activity
Measure by its ability to induce IL-8 secretion in human PBMCs. The ED50 for this effect is 1-5 ng/mL.
Purity
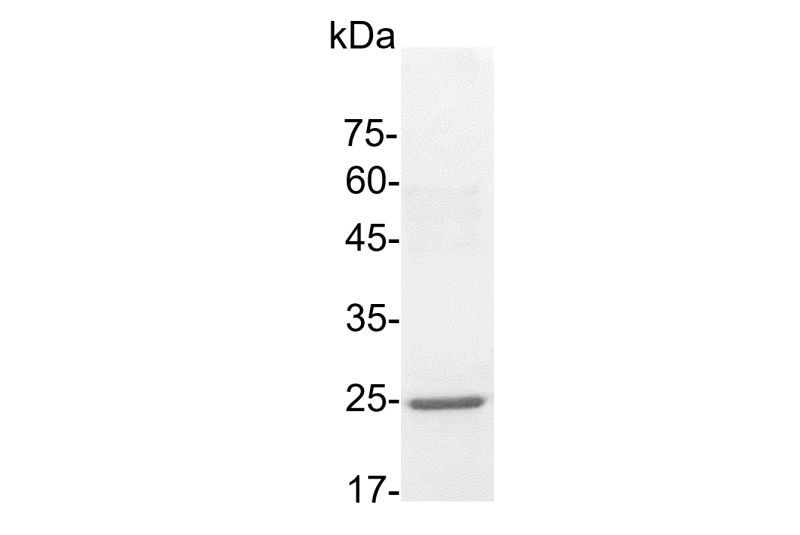
>95% as determined by SDS-PAGE analysis.
Form
Lyophilized
Storage Buffer
Lyophilized from a 0.2 μm filtered solution of PBS, pH 7.4.
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Stability & Storage
This product is stable after storage at:
• -20°C for 12 months in lyophilized state from date of receipt.
• -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Shipping conditions
Blue ice
Background Information
4-1BB Ligand Human Recombinant protein. 4-1BBL is a transmembrane cytokine that is part of the tumor necrosis factor (TNF) ligand family. Recombinant human 4-1BB ligand is intended for use in cell culture applications. 4-1BBL and its interaction with 4-1BB is involved in the antigen presenting process, proliferation of CD4 and CD8 positive T-cells, as well as cytokine secretion from T-cells.
Quality Statement
Croyez GMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP-certified facility.
The processes include:
● Animal-free reagent and laboratory
● Manufactured and tested under GMP guideline
● Testing and traceability of raw material
● Records of the maintenance and equipment calibration
● Personnel training records
● Batch-to-batch consistency
● Documentation of QA control and process changes
● Manufactured and tested under an ISO 13485:2016 certified quality management system
● Stability monitor of product shelf-life
Quality Assurance
At Croyez, we are committed to providing our valued customers with detailed product analytics to assist in their research endeavors. Our Certificate of Analysis includes the following lot-specific details:
● SDS-PAGE analysis and endotoxin level evaluation conducted on bulk QC lots.
● Lot-specific bioassay results ensuring compliance with established parameters, including microbial testing meeting USP <71> standards.
● Mycoplasma detection via PCR analysis.
We believe in transparency and strive to empower our customers with comprehensive information to aid in their decision-making process.
MREGPELSPDDPAGLLDLRQGMFAQLVAQNVLLIDGPLSWYSDPGLAGVSLTGGLSYKEDTKELVVAKAGVYYVFFQLELRRVVAGEGSGSVSLAL
HLQPLRSAAGAAALALTVDLPPASSEARNSAFGFQGRLLHLSAGQRLGVHLHTEARARHAWQLTQGATVLGLFRVTPEIPAGLPSPRSE
with polyhistidine tag at the C-terminus
UnitProt ID
P41273
Species of Origin
Human
Expression System
Escherichia coli
Endotoxin Level
<0.05 EU per 1 μg of the protein by the LAL method.
Activity
Measure by its ability to induce IL-8 secretion in human PBMCs. The ED50 for this effect is 1-5 ng/mL.
Purity
>95% as determined by SDS-PAGE analysis.
Form
Lyophilized
Storage Buffer
Lyophilized from a 0.2 μm filtered solution of PBS, pH 7.4.
Reconstitution
It is recommended to reconstitute the lyophilized protein in sterile H₂O to a concentration not less than 0.5 mg/mL and incubate the stock solution for at least 20 min to ensure sufficient re-dissolved.
Stability & Storage
This product is stable after storage at:
• -20°C for 12 months in lyophilized state from date of receipt.
• -20°C or -80°C for 1 month under sterile conditions after reconstitution.
Avoid repeated freeze/thaw cycles.
Shipping conditions
Blue ice
Background Information
4-1BB Ligand Human Recombinant protein. 4-1BBL is a transmembrane cytokine that is part of the tumor necrosis factor (TNF) ligand family. Recombinant human 4-1BB ligand is intended for use in cell culture applications. 4-1BBL and its interaction with 4-1BB is involved in the antigen presenting process, proliferation of CD4 and CD8 positive T-cells, as well as cytokine secretion from T-cells.
Quality Statement
Croyez GMP® recombinant proteins are manufactured in ISO 13485:2016 and GMP-certified facility.
The processes include:
● Animal-free reagent and laboratory
● Manufactured and tested under GMP guideline
● Testing and traceability of raw material
● Records of the maintenance and equipment calibration
● Personnel training records
● Batch-to-batch consistency
● Documentation of QA control and process changes
● Manufactured and tested under an ISO 13485:2016 certified quality management system
● Stability monitor of product shelf-life
Quality Assurance
At Croyez, we are committed to providing our valued customers with detailed product analytics to assist in their research endeavors. Our Certificate of Analysis includes the following lot-specific details:
● SDS-PAGE analysis and endotoxin level evaluation conducted on bulk QC lots.
● Lot-specific bioassay results ensuring compliance with established parameters, including microbial testing meeting USP <71> standards.
● Mycoplasma detection via PCR analysis.
We believe in transparency and strive to empower our customers with comprehensive information to aid in their decision-making process.
Downloads
→ Materials For iPSC differentiation
Selected Scholarly Works from Network for Your Insight:
→ 4-1BBL as a Mediator of Cross-Talk between Innate, Adaptive, and Regulatory Immunity against Cancer
→ T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection
→ 4-1BB: A promising target for cancer immunotherapy
→ Role of 4-1BB:4-1BB ligand in cancer immunotherapy
→ 4-1BB-4-1BBL cis-interaction contributes to the survival of self-reactive CD8+ T cell
→ Limited Cross-Linking of 4-1BB by 4-1BB Ligand and the Agonist Monoclonal Antibody Utomilumab